
We recently announced that our groundbreaking new approach to pancreatic cancer clinical trials – PanCAN’s Precision PromiseSM – is open and enrolling eligible patients at world-class cancer institutions across the country. We created Precision Promise to speed progress for patients by getting new, better treatments approved more quickly.
PanCAN’s Precision Promise is:
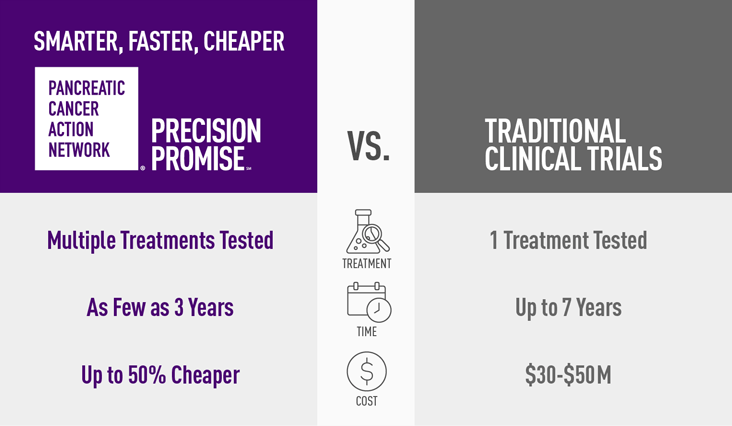
- Faster – researchers will learn in two to four years, not eight to 10 as with standard clinical trials
- Cheaper – PanCAN’s Precision Promise is up to 50% less expensive to operate than a traditional clinical trial
- Smarter – clinicians learn from every single patient every step of the way, and modifications can be made throughout the trial
We sat down with PanCAN President and CEO Julie Fleshman, JD, MBA, to discuss the path to Precision Promise, which was four years in the making.
Today’s story is part 1 of 2 on the bold path to bringing Precision Promise to life.
Q: Creating Precision Promise was a significant undertaking for PanCAN. Why was it critical?
JF: Simply put, progress for pancreatic cancer patients has not been fast enough. New treatment options are needed in order for patients to live longer, and those haven’t been happening at the pace we’d like to see.
Clinical trials are the only way to get new treatments approved, and there are challenges with the traditional clinical trial process. It’s costly, it’s slow and the results from trials don’t necessarily build on each other – the field hasn’t been learning from them like it should.

Julie Fleshman, JD, MBA, PanCAN’s President and CEO
There’s the saying that if you keep doing the same thing over and over, you’re going to get the same results. At PanCAN, it’s in our nature to take risks, and we’ve never shied away from trying something different. So, we began thinking about a new approach to pancreatic cancer clinical trials, something disruptive.
We had several goals – to create a clinical trial design where each finding informed the next one, that learned from every patient and that put the patient at the center of every decision. A design that took less time and required fewer patients to test a treatment, and a design that would cost less money so it could de-risk pancreatic cancer drug development for pharmaceutical companies.
Most importantly, our number-one goal was – and still is – to transform outcomes for pancreatic cancer patients.
Q: There were a few important and timely factors that also highlighted the need – tell us about that.
JF: We had set a goal to double survival for pancreatic cancer by 2020, and we took that goal very seriously as we thought about what it would take to truly accelerate progress.
With that as our backdrop, we had just published a paper looking at the last 30 years of Phase II and Phase III pancreatic cancer clinical trials. The paper concluded that the process was slow, the field wasn’t learning from every patient on the trial, some trials progressed to Phase III when maybe they shouldn’t have because Phase II data wasn’t properly considered, and that overall the success of new pancreatic cancer treatments has been rather dismal at only 10%.
We were also starting to think about the role of biomarker testing in pancreatic cancer. At the time, biomarker testing of each individual’s tumor wasn’t commonly being done for pancreatic cancer patients to help determine the best treatment options.
PanCAN wanted to find out if doing biomarker testing was beneficial for pancreatic cancer patients. Our overall attitude was with more information, let’s see if we can better identify treatment options for individual patients.
Our Know Your Tumor® service was helping us learn more about this. It also illustrated that PanCAN could take on a big clinical initiative that involved patient consent, collection of data and collaboration among different stakeholders.
Q: What happened next?
JF: After we published our paper analyzing the clinical trial landscape and saw the early success of our Know Your Tumor service, we began to discuss the idea of a new approach (which would become Precision Promise) to clinical trials with a team of experts from across the country.
We met with researchers, clinicians, leaders in industry/pharmaceuticals and others, to discuss what this could look like and how to make it happen. Today there are more than 100 experts that we have worked with to bring Precision Promise to life.
Q: What made PanCAN the right organization to start this conversation?
JF: Over two decades, PanCAN has built a reputation as an unbiased industry leader – an organization that brings multiple stakeholders from many disciplines together to make progress for patients. And over the years, we’ve proven we can get things done that were previously thought not possible. Our Know Your Tumor service was one of those things.

Julie Fleshman speaks with researchers and healthcare professionals, among them members of PanCAN’s Precision Promise Steering Committee, at PanCAN’s Scientific Summit 2019.
So we are uniquely positioned to lead a project of this magnitude.
It wouldn’t have been possible for any single institution or organization to take this on alone, and PanCAN was the right organization to convene a team of experts for the level of collaboration needed.
Our advisors have invested an enormous amount of time from the start of those initial conversations until now, and I am extremely grateful to all of them. We all knew that this was absolutely the right thing to do for patients.
Q: PanCAN’s comprehensive approach to accelerating progress for patients also provided a solid foundation on which Precision Promise was built. Tell us more.
JF: PanCAN was the first nonprofit organization dedicated to fighting pancreatic cancer in a comprehensive way.
Within a few years of our founding, we created a one-on-one support service for patients and families – a place to go for free information, resources to help guide them every step of the way after diagnosis, and maybe most importantly, hope.
Today we speak with more pancreatic cancer patients and families than any other organization in the world through our Patient Services program. From speaking to patients and their families now for 20 years, we understand what patients want. This understanding provides us the opportunity to represent the patient’s voice, but also to ensure that new clinical approaches are shared with patients and families so that they can make informed decisions about their own care and treatment.
Precision Promise builds on this foundation that is rooted in keeping the patient at the center of everything we do and with a strong commitment to accelerating progress.
For more information about Precision Promise and other clinical trials, as well as free, in-depth and personalized resources for pancreatic cancer, contact PanCAN’s Patient Services.
Next week: The Bold Path to Precision Promise, Part 2. Read more about how PanCAN set out to transform standard clinical trials for pancreatic cancer patients, including challenges, lessons learned and how we’ll measure success.





