
On World Pancreatic Cancer Day, Nov. 19, 2020, the Pancreatic Cancer Action Network (PanCAN) hosted a webinar titled, “Treatment Advances: The Latest in Pancreatic Cancer Clinical Research.”
The virtual event was hosted by Julie Fleshman, JD, MBA, PanCAN’s president and CEO. Fleshman opened by telling listeners, “These are all the amazing things that you make possible.”
PanCAN funds transformative research across the country – everything from early detection to new treatment approaches. Thanks to our passionate supporters, we’ve invested approximately $126 million in pancreatic cancer research since 2003, and we will invest another $23 million this year.
Fleshman was joined by a prestigious panel of experts, including PanCAN’s chief science officer, Lynn Matrisian, PhD, MBA, and oncologists Thomas George, MD, FACP, and Andrew Hendifar, MD, MPH.

Below are key takeaways from the webinar:
The pancreatic cancer research and clinical trial field are growing.
When PanCAN was founded in 1999, Matrisian said, there were about 30 researchers across the United States who were funded by the government to study pancreatic cancer. Today, 20 years later, there are more than 330!
These researchers will make the breakthroughs and advances that we want to see – a better understanding of the biology of pancreatic cancer will help detect, treat, prevent and manage the disease.
Matrisian also spoke about the growth within the clinical trial space. There are more than 150 pancreatic cancer clinical trials enrolling patients throughout the country, testing investigational therapies aiming to improve patient outcomes. Encouragingly, more trials than ever before are evaluating treatments offered to patients as their second, third – or beyond – type of treatment. This indicates patients are living longer and are feeling well enough to continue trying investigational therapies – and that options are available to them.
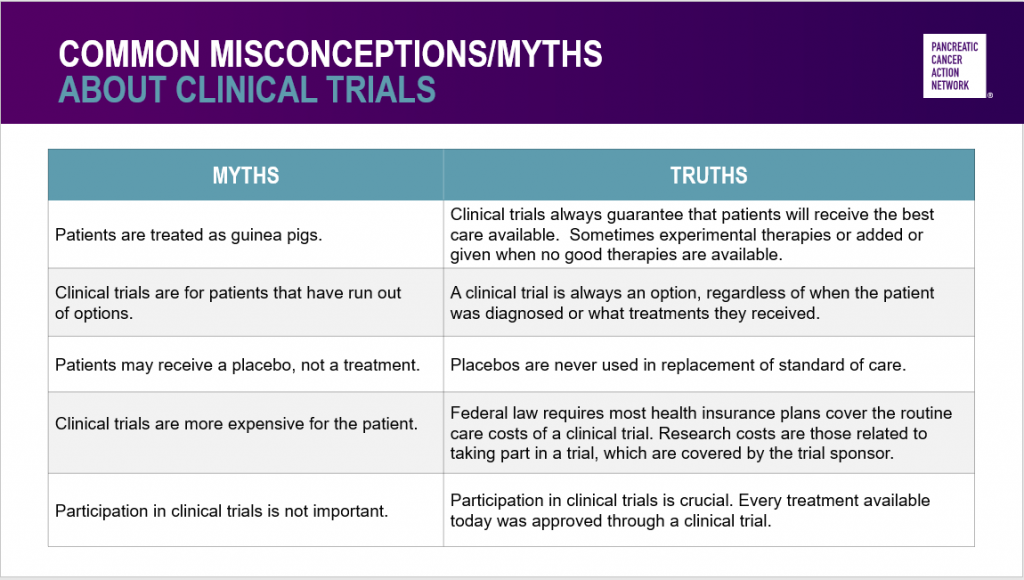
Hendifar followed up with some common myths about clinical trials – and emphasized that clinical trials are safe and critically important.
Attacking cancer metabolism is a promising approach.
Healthy cells within our body need energy to grow and survive. That energy primarily comes from oxygen in the air we breathe and nutrients from the food we eat – and is derived through a process called metabolism.
But we know that in pancreatic tumors the cancer cells are very rapidly growing and are surrounded by dense tissue known as the stroma. Because of this, they have a very limited supply of oxygen and nutrients.
Through evolution, cells have developed what Matrisian described as “emergency pathways” to get enough oxygen and nutrients to make the energy needed to repair cellular damage, survive and grow.
Therefore, stopping these emergency pathways has the potential to starve and suffocate the cells and cause cell death. Clinical trials are currently underway to evaluate investigational treatments targeting pancreatic cancer cell metabolism.
Patients can receive personalized treatment options.
Historically, cancer patients were treated uniformly – standards of care were determined by the tumor’s location in the body, without considering its biological features.
Today, Hendifar described, treatment options have changed dramatically. Factors like the patient’s tumor biology and genetic makeup are routinely factored into treatment options offered to them.
Learnings from PanCAN’s Know Your Tumor® precision medicine service have shown that one out of every four pancreatic cancer patients’ tumors has at least one alteration – or biological change – that can indicate a certain type of treatment may work well for them. And excitingly, those patients who are able to go on treatment that matches their tumor biology live an average of one year longer than those who can’t.
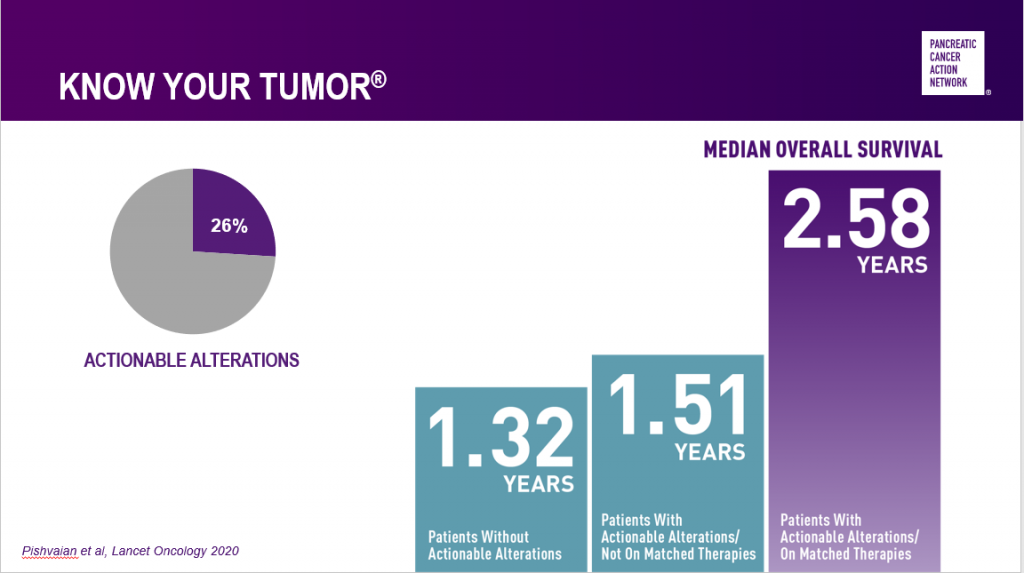
The only way for patients to know whether their tumor biology or genetic makeup aligns with certain treatment options is through testing – biomarker testing of tumor tissue and genetic testing for inherited mutations of blood or saliva. Both tests are available through PanCAN’s Know Your Tumor service, and our Patient Services team can provide additional information about other ways to access the tests.
Immunotherapy is a “major area of hope.”
George spoke about immunotherapy – or harnessing the patient’s own immune system to recognize, find and kill cancer cells – as the “holy grail” and a “major area of hope.”
Immunotherapy has already revolutionized treatment for some cancer types, like melanoma. With these cancer types, the immune system is poised and ready to attack the cancer cells. However, the tumor has ways to hide itself and evade an attack. Once those mechanisms are disabled, the immune system can effectively hunt and kill the cancer cells within the body.
However, immunotherapy drugs that have brought great success to other cancer types have not been effective in pancreatic cancer yet.
The reason, George explained, is that pancreatic tumors are “insanely good” at hiding from the immune system.
Immunotherapy approaches being investigated for pancreatic cancer patients now include complex combinations, including standard treatments like chemotherapy and radiation, along with investigational therapies that can turn on the immune system while exposing the tumor to immune attack.
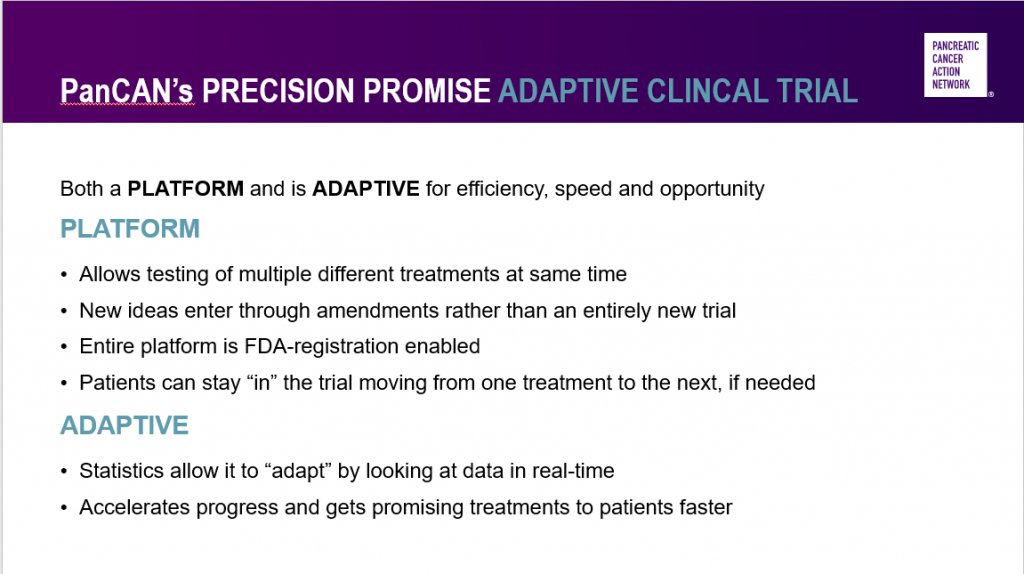
Adaptive platform clinical trials – like PanCAN’s Precision PromiseSM – are the future of clinical research.
“Precision Promise is one of the most innovative, cutting-edge clinical trials for pancreatic cancer patients that I’ve ever seen,” George said.
The platform aspect of Precision Promise means that multiple investigational drugs will be tested at the same time. George emphasized that this allows new investigational drugs to enter the trial quickly and more easily than developing an entirely new clinical trial to test them.
Also, the U.S. Food and Drug Administration (FDA) has approved the Precision Promise clinical trial design so that drugs that are successful in the trial can go straight to review for FDA approval. This will lead to new treatment options available to more patients faster.
Precision Promise is also adaptive – statisticians will constantly analyze the data and determine which investigational treatments hold the most promise. Unsuccessful drugs will be removed from the trial, while more patients will be assigned to the most promising treatment options.
In addition to testing investigational drugs compared to standard of care options, Precision Promise will include robust supportive care for patients.
“I’ve never been involved with a clinical trial that has put more focus and dedication on how patients actually feel,” said Hendifar, who chairs the Precision Promise Supportive Care Committee.
Supportive care through Precision Promise will include nutritional support, measurement of patients’ activity levels, focus on sleep patterns and more.
“PanCAN is so proud to sponsor this groundbreaking clinical trial,” Fleshman said. “It’s thanks to our donors who are allowing us to fund this trial and make it possible for patients today and in the future to have access to new, better treatment options.”






